Secure, fast, and easy – Bitcoin payments are now live on our site!
You can now order your favorite in-stock products online—quick, simple, and hassle-free!
Secure, fast, and easy – Bitcoin payments are now live on our site!
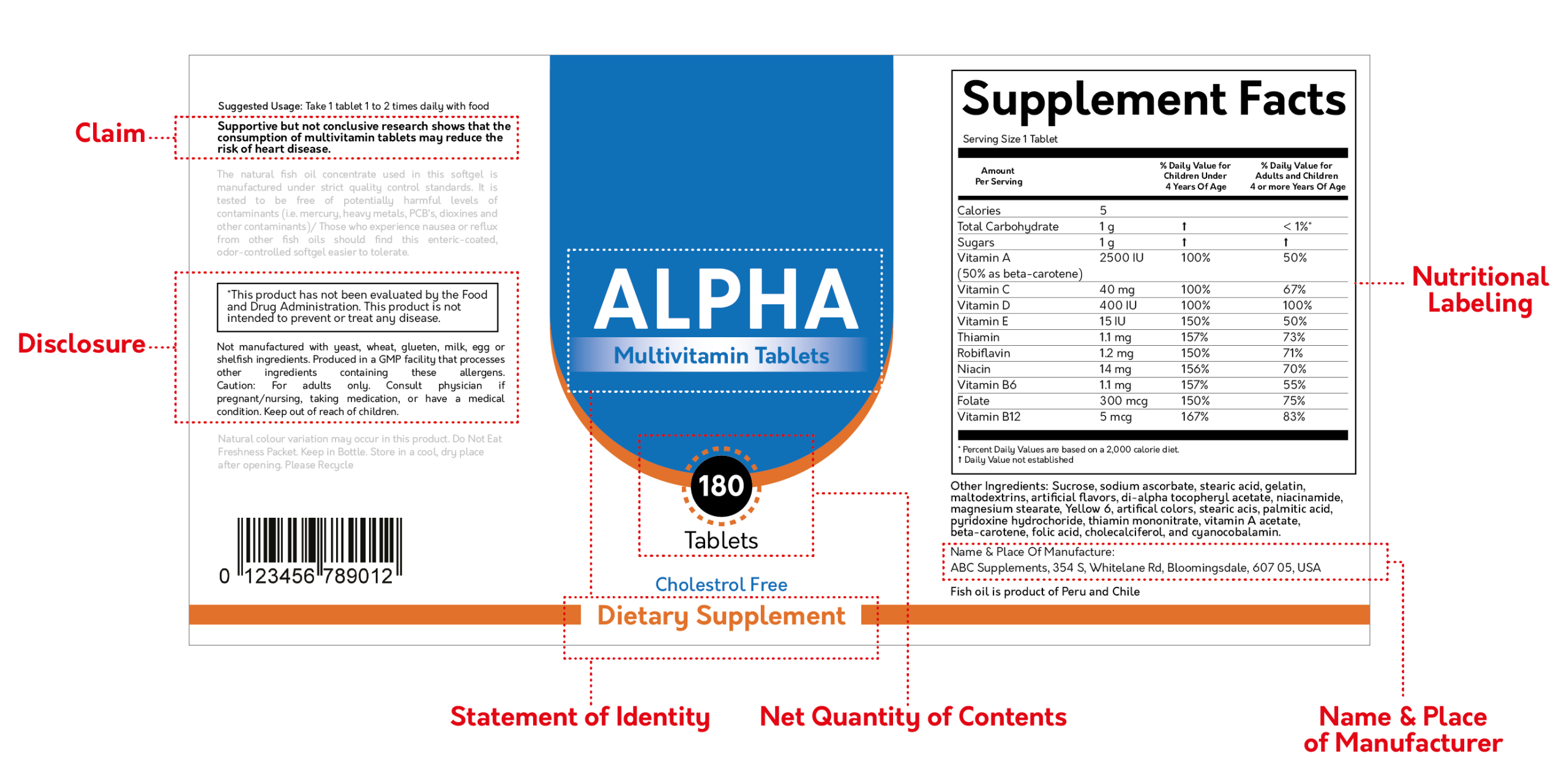
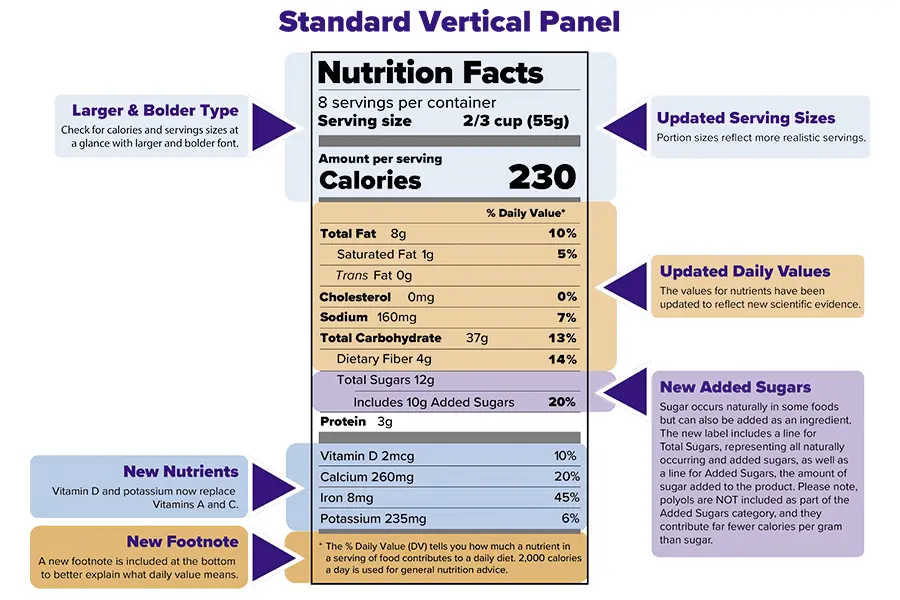
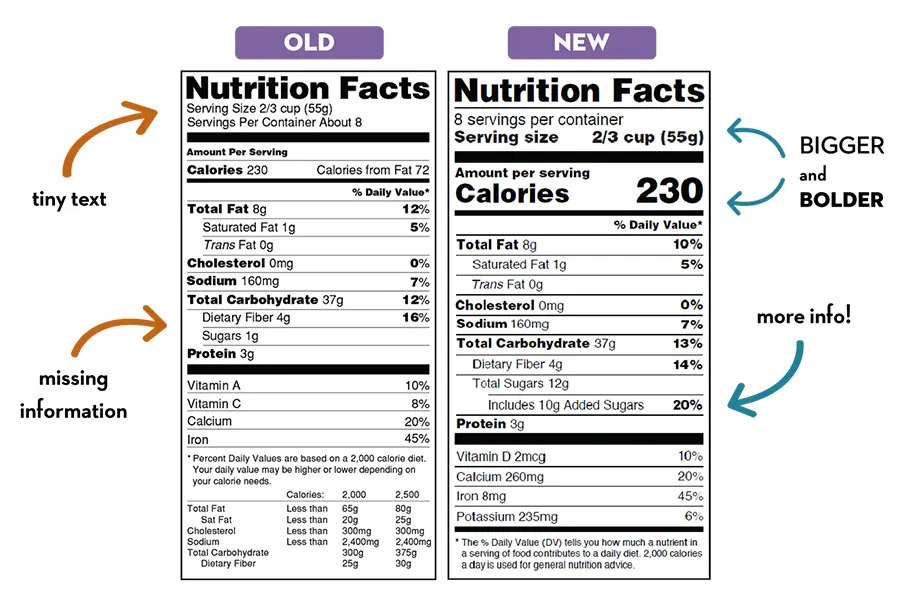
Please note: A $100.00 QA/FDA label review fee will apply if your labels are not designed by Vitakem.
Secure, fast, and easy – Bitcoin payments are now live on our site!
You can now order your favorite in-stock products online—quick, simple, and hassle-free!
Secure, fast, and easy – Bitcoin payments are now live on our site!
Please note: A $100.00 QA/FDA label review fee will apply if your labels are not designed by Vitakem.